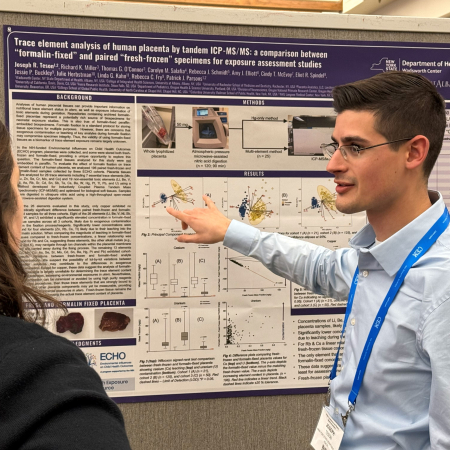
Wadsworth Scientists Present Research at the Winter Conference on Plasma Spectrochemistry
Several Wadsworth Center Research Scientists and their trainees attended the 2026 Winter Conference on Plasma Spectrochemistry, held January 10-17, 2026, in Tucson, Arizona. Participants included Drs. Patrick Parsons, George Donati, and Christopher Palmer; Ms. Kayla Mehigan; and Dr. Sarah El Din, an APHL Postdoctoral Fellow. University at Albany doctoral students Deanna Luneau and Joseph Teson from Dr. Parsons’ laboratory also attended the meeting.
READ MORE about Wadsworth Scientists Present Research at the Winter Conference on Plasma Spectrochemistry